
Sometimes influences to quit smoking come from the most unusual sources. Usually these voices are muted in comparison with the trumpet of tobacco trade, but nonetheless a small voice—that of a grandson of a smoking grandparent or the inner voice of a pregnant woman—can change what years of habit and good intentions cannot. In the paragraphs below we are going to discuss special populations in the context of smoking—the unborn and young who are at risk for exposure to tobacco's effects because a mother or other household member smokes, middle school and high school students who get hooked on smoking early, those who are genetically predisposed to tobacco use, and individuals who are depressed.
These special populations who are vulnerable to tobacco addiction or to the harm tobacco can cause demand special considerations from those interested in promoting cessation. Their unique situations require recognition, especially inasmuch as they may require special attention or tailored interventions that might not be necessary for others who are seeking to quit. Experience and familiarity with these cases may eventually equip physicians, pharmacists or others with skills specially suited to meeting the particular needs of these groups and make them "specialists" in their care.
Though smokers can be heard defending their habit by saying it doesn't affect anyone but them, that is clearly a claim pregnant women and parents cannot make. Smoking among pregnant women and the parents of young children affects not only the adults but also the children, whose developing and sensitive respiratory tracts make them especially vulnerable to tobacco's harm.
The potential adverse effects on fetal development are what make smoking among women of child-bearing age an important issue. The consequences of smoking during pregnancy include the following life-threatening risks:
Maternal smoking is associated annually with the birth of up to 61,000 babies born with low birth weight and up to 26,000 admissions to neonatal intensive care units. In 1999 about 12.3% of women who gave birth were smokers, marking a 10-year decline in maternal smoking. An exception to this general rule was found in the group of girls 15 to 19 years of age. In that group, the percentage that smoked declined the first half of the period (between 1990 and 1994), but it grew between 1995 and 1999. At the close of the 1990s, 17.5% of women 15–19 years of age who were pregnant smoked. That rate makes this age group the one with the highest rate of smoking. The group with the largest decline between 1990 and 1999 was made up of women 30–34 years of age. In this group, smoking rates were cut almost in half between 1990 and 1999.
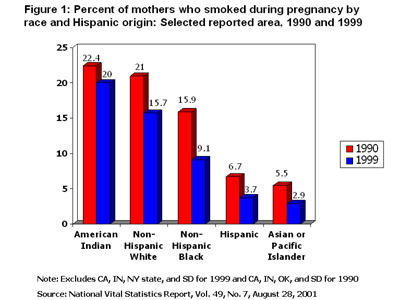
Women who were of native American Indian descent or were non-Hispanic whites were those most likely to have smoked during pregnancy. Though smoking rates declined in these groups between 1990 and 1999 (about 10% in American Indians and 25% in non-Hispanic whites), they remained above the overall rate in 1999: 20.0% of American Indians smoked, and 15.7% of non-Hispanic whites smoked. Groups with the lowest pregnancy smoking rates were Hispanics (3.7%) and Asian or Pacific Islanders (2.9%) (Fig. 1).

Two subgroups approach or surpass the national rate. A subgroup of Hispanics, Puerto Ricans, represents a spike in the rates, with 10.5% of this group smoking during pregnancy, seven times the low 1.4% found among other Hispanic subgroups (Central and South Americans). Rates were also high in Hawaiian women, 14.7% of whom smoked during pregnancy (Fig. 2).

Other factors affecting maternal smoking include residence in a tobacco- producing state and education level. Maternal smoking rates were highest among those without a high school education (29%) and lowest among those with four or more years of college (2%). The two states with the highest maternal smoking rates were West Virginia and Kentucky (26.1% and 24.5%, respectively), both tobacco-producing states.
To combat the ills that affect the unborn because of smoking during pregnancy, the federal government recommends interventions throughout the life course of women. This is because months can pass before a woman knows she is pregnant and becomes willing to quit to protect her baby even if she was formerly unwilling to quit to protect herself.
Interventions for women who are of child-bearing age or are pregnant should take into account these differences. Behavioral approaches to tobacco cessation are preferred for pregnant women because clinical studies of bupropion in pregnant women have not been done. Classified as a category B drug, bupropion is recommended by its manufacturer for use in pregnancy only if clearly needed (Table 1).
| Table 1: U.S. Department of Health and Human Services Clinical Practice Guideline, Treating Tobacco Use and Dependence |
| Recommendations During Pregnancy |
| Recommendation: Because of the serious risks of smoking to the pregnant smoker and the fetus, whenever possible pregnant smokers should be offered extended or augmented psychosocial interventions that exceed minimal advice to quit. (Strength of evidence = A) |
| Recommendation: Although abstinence early in pregnancy will produce the greatest benefits to the fetus and expectant mother, quitting at any point in pregnancy can yield benefits. Therefore, clinicians should offer effective smoking cessation interventions to pregnant smokers at the first prenatal visit as well as throughout the course of pregnancy. (Strength of evidence = B) |
| Recommendation: Pharmacotherapy should be considered when a pregnant woman is otherwise unable to quit, and when the likelihood of quitting, with its potential benefits, outweighs the risks of the pharmacotherapy and potential continued smoking. (Strength of evidence = C) |
For children whose parents' tobacco habit persists into their early years, the consequences can be serious. Smoking while breastfeeding exposes infants to harmful chemicals. Effects of heavy smoking during breastfeeding can include reduction of the mother's milk supply and nausea, abdominal cramps, vomiting, and diarrhea in the infant.
Children's exposure to second-hand smoke is associated with recurring ear infections and colds and more serious ailments, including:
Interventions for those of child-bearing age or for parents often focus on the benefits to the unborn or the children. These arguments can prove persuasive and powerful (Table 2).
| Table 2: U.S. Department of Health and Human Services Clinical Practice Guideline, Treating Tobacco Use and Dependence | |
| Clinical Practice | Rationale |
|
Assess pregnant women's tobacco use status using a multiple-choice question to improve disclosure. |
Many pregnant women deny smoking, and the multiple-choice format improves disclosure. For example: "Which of the following statements best describes your cigarette smoking?"
|
|
Congratulate those smokers who have quit on their own. |
To encourage continued abstinence. |
|
Motivate quit attempts by providing educational messages about the impact of smoking on both the woman's and the fetus' health. |
These are associated with higher quit rates. |
|
Give clear strong advice to quit as soon as possible. |
Quitting early in pregnancy provides the greatest benefits to fetus. |
|
Suggest the use of problem solving methods and provide social support and pregnancy-specific self-help materials. |
Reinforces pregnancy-specific benefits and ways to achieve cessation. |
|
Arrange for follow-up assessments throughout pregnancy, including further encouragement of cessation. |
The woman and her fetus will benefit even when quitting occurs late in pregnancy. |
|
In the early postpartum period, assess for relapse and use relapse prevention strategies recognizing that patients may minimize or deny. |
Postpartum relapse rates are high even if a woman maintains abstinence throughout pregnancy. Relapse prevention may start during pregnancy. |
Imagine the high school in your neighborhood. If you live in a city, it is possible that you will find 3,000 students in that school. Now imagine that one day, every one of the students in that school became a smoker. If you can imagine that, then you have an idea of how many adolescents become regular smokers every day in the United States.
In the United States, more than six million adolescents are smokers, or almost 30%. In Texas, about 10% of middle school students and 33% of high school students smoke. Overall, teen smoking rates are higher in Texas than they are in the nation. The percentage of high school students who smoke is 28.4% nationwide, and it is 32.8% in Texas. Rates among high school teens are also higher when measures go beyond smoking to encompass use of any type of tobacco (34.8%, nationwide; 42.1%, Texas). Within Texas, teen use of any tobacco is generally higher in boys than in girls, and it rises more dramatically in boys than in girls between middle school and high school. Use of any tobacco was found to be 18.7% in girls and 27.1% in boys in grades 6 to 8 in a 2002 report, but it rose to 35.7% in girls and 48.1% in boys in grades 9 to 12.
The good news from this 2002 report is that the prevalence of current tobacco use among Texas middle school and high school teens declined between 1999 and 2001. Overall, this was true across all grades 6 through 12, across both sexes, and across the three major racial and ethnic groups (white, African-American, and Hispanic). A few spikes, however, were found in subsets. Notable were an increase in cigar use by Asian children in middle school and in current cigarette use by Asians in high school Hispanic teens also reported data indicating an increase in cigar smoking and pipe smoking, although overall rates were about 12% and 5%, respectively, and increases were less than a percentage point. Increases were similarly small among African-American teens in smokeless tobacco (high school) and pipe use (middle school and high school).
While they are in high school, adolescents who smoke believe that in the future— say, five years after graduation—they will not be smoking—but, in fact, 75% of them are still smoking at that time. Clearly, teens find it difficult to quit, with 84% of smokers 12 to 17 years of age in one study reporting they "needed" or "were dependent" on cigarettes, and in another, fewer than 20% were able to remain free of tobacco for more than one month. In one study of girls in Britain, researchers found that 74% of girls who smoked daily and 47% of girls who smoked occasionally (63% overall) reported withdrawal symptoms similar to those of adults when they try to quit. Smokers with four or more withdrawal symptoms—dysphoria, anxiety, irritability, difficulty concentrating, restlessness, increased appetite, or insomnia—are identified as experiencing nicotine withdrawal symptoms. Some of these teens, who when they started smoking may have believed they were " too young to be hooked," discovered differently.
The seriousness of an adolescent's nicotine dependence is a significant factor in his or her ability to become tobacco free. Nicotine dependence has been rated in teen smokers from 20% to 70%, with such variables as the age of the population, the number of cigarettes smoked each day, the instrument used to measure nicotine dependence, and the scoring criteria affecting the measure. In the mid 1990s, researchers at The University of Texas M. D. Anderson Cancer Center adapted for adolescents the Fagerström Tolerance Questionnaire, which had been developed in 1978 to gauge adult dependence. Simple and quick to administer and score, this seven-question test has been psychometrically and biologically validated, and its test-retest reliability confirmed.
The nicotine dependence the test measures provides an index to teens' readiness to quit and the difficulty they will encounter when they attempt to quit. Time is not on the side of these young smokers, because the longer they smoke, the greater their dependence, and the greater their dependence, the less likely they are to be ready to attempt to quit. Findings also indicate that failing at quitting was likely to lead adolescents to give up trying, which is especially distressing inasmuch as research has shown that, on average, most successful cessation attempts come after four or five failures. It is not surprising, then, that success is rare: only 1 teen smoker in 50 who attempts to quit does so successfully.
The costs of teen addiction are great. Theoretically, teens who start smoking at 15 and pick up a pack-a-day habit will, if they smoke till age 65 years and pay $3.75 per pack, spend $363,423 over 50 years. If that habit is a three pack–a–day habit, the cost is well over $1 million. This does not also take into account the costs of lost productivity— approximately $3.75 per pack—and medical costs attributable to smoking—$3.45 per pack. Add to this the estimated years of life lost, which for men is 13.2 years and for women is 14.5 years. Unestimated remain the frustration and depression associated with a lifetime of smoking, which, like other costs associated with early smoking, are great.
Despite these high economic and emotional costs, selling to teens, say critics, remains a major aim of the tobacco companies, even though they agreed as part of the 1998 tobacco settlement with states to reduce advertising and promotional programs aimed at teens. The New England Journal of Medicine reported that two years later, in 2000, tobacco companies spent $59.6 million in advertising their most popular youth brands in magazines intended for the young. In fact, these same researchers estimated that more than 80% of U.S. youth were reached by tobacco ads an average of 17 times in 2000.
It's been said that tobacco companies are "addicted to underage smoking." To prove the point, critics point to research indicating that initiation of smoking and promotion of the smoking habit are most easily achieved in children and adolescents, making them easy prey for the cigarette manufacturers:
These results come from government studies reported by the Substance Abuse and Mental Health Services Administration (SAMHSA) and other agencies of the U.S. government and research published in the Journal of Marketing and other professional and medical journals.
The power of teen addiction, the multiple attempts required to quit, and the pervasive advertising and promotion make escaping tobacco use a daunting challenge to teens. Experts who help adolescents quit say that interventions matched to the adolescents' stage of change are most effective. To identify the adolescent's readiness to quit—his or her stage of change—health care professionals can use the following questions:
...and then follow the suggestions listed below appropriate to the patient's stage.
Figure 3 below diagrams the process.
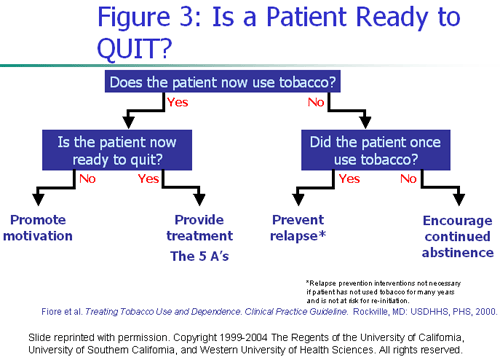
Interventions that make quitting more appealing to teens include those that de- glamorize smoking and emphasize the realities of the irritating smoke and foul smell and the resulting yellow teeth and bad breath. Like adult smokers trying to quit, adolescents need skills at handling the urge to smoke in familiar situations and advice about modifying lifestyle. Teens in maintenance remain vulnerable to relapse, so health care professionals are urged to follow them mindfully, offering support and suggestions meant to prevent relapse.
Almost fifty years ago, a researcher suggested that there might be a genetic predisposition to smoking and lung cancer that explained why teens began smoking and why their lungs later, when they were adults, were found to have tumors. Today we know that genetic factors do affect vulnerability to tobacco use and dependence, but we also know that the genes that predispose to dependence are not the same ones that cause lung cancer.
To learn more about genetic predisposition to smoking and to separate genetic factors from environmental influences, researchers have relied on studies of twins reared apart, adopted children, and genome mapping; compared adopted sibling pairs to biological sibling pairs; and investigated genes thought to be candidates for explaining susceptibility to smoking dependence. What they have found is that genes appear to explain tobacco dependence in part, but that more research is needed to understand the mechanisms at work.
Researchers have found, for example, that there are stronger associations between family members and the number of cigarettes consumed than there are between individuals who are not biologically related and the average number of cigarettes consumed. Correlation coefficients ranged from 0.52 for identical twins and 0.30 for fraternal twins to 0.05 for adoptive siblings and -0.02 for adoptive parents and adoptive offspring. In addition, research in families has consistently shown that family members of smokers are more likely to be smokers than are family members of nonsmokers. Though it must be understood that environmental effects were not accounted for in the research design, one study showed that people with a nicotine-dependent sibling were 2.1 to 3.5 times more likely to also be nicotine dependent than were those who did not have a nicotine-dependent sibling.
In twin studies, researchers reasoned that identical twins, because they have greater genetic concordance, are likely to have greater concordance in their tobacco habits than are fraternal twins. Results supported researchers' hypotheses: identical twins did demonstrate greater concordance in their tobacco use behavior than did fraternal twins. Tobacco use included being a nonsmoker, smoking, quitting smoking, and the amount smoked. Furthermore, they estimated average heritability of smoking to be 0.53 (range, 0.28–0.84); therefore, the researchers concluded that about half of the variance in smoking is owed to genetic factors. Kendler, Thornton, and Pederson (2000) reported similar findings in a study of twins reared apart. Studying these highly genetically concordant twins reared in different environments allowed researchers to more reliably differentiate between environmental and genetic influences. They attributed to genetic factors 60% of the variance in regular smoking in men and women born after 1940.
Research here at M. D. Anderson Cancer Center in genetic factors affecting tobacco use includes work by Drs. Cinciripini, Spitz, and Wu who with colleagues examined the prevalence and functional significance of polymorphisms in the DRD2 gene as part of ongoing work. They found that the B1 allele was more prevalent in current and foreign smokers than in nonsmokers. Later work is thought to be the first study to show a link between participants' genetic background and response to an antidepressant drug.
These are just a few of the studies under way to define better the link between genetics and tobacco use. This other initial work is beginning to reveal findings with implications for cessation interventions:
Health professionals face distinct and substantial obstacles in attempting to help smokers who are depressed to initiate an effort to quit smoking and follow through. Depression and smoking pose somewhat of a chicken-and-egg question, but determining which came first doesn't seem as important to researchers currently as determining the effects each has on the other and the effect depression has on quitting smoking. Major depression is more than twice as common among smokers as it is among nonsmokers, depressive mood is more common among smokers than among nonsmokers, and studies show that smokers who are depressed find it more difficult to quit than smokers who aren't.
In one study major depression was found in 6.6% smokers but only 2.9% of nonsmokers ; moreover, in a study of adolescents free of notable depressive symptoms at baseline, researchers found that 18% of "established" smokers reported depressive symptoms, whereas only about half as many— 9.8%—of nonsmokers reported such symptoms.
Investigators have found that depression is closely linked to nicotine-dependent smoking, that smokers with depressive symptoms are more likely to experience more severe withdrawal and withdrawal-induced depressive symptoms, and that such symptoms are associated with a decreased likelihood of success at quitting. In particular, Cinciripini and colleagues at The University of Texas M. D. Anderson Cancer Center found that having a depressed mood before attempting cessation was inversely related to successful six-month abstinence.
In effect, depression appears to short-circuit cessation efforts: only 22% of nondepressed women smoked on the first day of abstinence during a cessation attempt, but 48% of depressed women did. Furthermore, study subjects in another trial who were without symptoms of depression delayed relapse a median of 30 days, but subjects who had even one symptom of depression aborted their cessation attempt at a median of 3.5 days. However, the relationship between a history of major depression and cessation success has not always been clear-cut in clinical samples. Some studies have shown that having a history of depression does not necessarily lead to a poorer treatment outcome.
Negative affect is a term that refers to a composite of depression, dysphoria, irritability, nervousness, and other negative moods. Negative affect has been duly linked with smoking initiation and relapse. Researchers have found a positive correlation between the likelihood of being a smoker and rising levels of daily negative mood, whether it is depression, loneliness, boredom or other similarly negative mood that is experienced. Negative affect also been identified as a factor in more than 50% of all smoking cessation lapses, and as with depression alone, negative affect strongly predicts a failure to quit or a predilection to return to smoking after a short attempt at abstinence.
Although investigators have aided behavioral scientists interested in understanding the power of negative affect to influence smoking habits and cessation attempts, they are only beginning to conduct studies that specifically target the relationship in interventions. Recognizing that modulation of mood is an important reason why people smoke, the panel devising clinical practice guidelines for tobacco cessation recommended one antidepressant, buproprion SR, as a first-line therapy, and one other, Nortriptyline as a second-line pharmaceutical intervention. Buproprion is the only antidepressant approved by the U.S. Food and Drug Administration (FDA) for treatment of tobacco dependence.
In behavioral intervention efforts, Hall and colleagues determined that mood management provided a differentially favorable effect in increasing abstinence among those whose medical history included a bout with depression, and other investigators have employed their methods with some success with smokers who have a history of depression. This effect was primarily due to the poor performance of smokers who received the control as opposed to the mood management intervention. Cinciripini and coworkers found that self-efficacy was the strongest mediator of the effects of depressed mood on abstinence during the post cessation period, accounting for up to 48% of the effect of mood on abstinence. They concluded that building the smoker's sense of self- efficacy during the intervention, particularly during the early stages, may be a significant factor in successful perseverance during the quit attempt.
These reports have led investigators to agree with Anda et al., who concluded more than a decade ago that depression plays an important role in the dynamics of cigarette smoking. Part of what remains to be revealed by future research is the scope of that role and, also, a better delineation of depression's role in the dynamics of cessation.
Smoking's effects stretch beyond the individual who smokes to affect the lives of those around him or her, including the unborn and the very small. What we know now is that reasons for smoking may be sown into the smoker's genetic code early on and that only more research will begin to delineate the mechanisms responsible for nicotine dependence and other characteristics of the smoking habit. As more of this information becomes known, it may well transform our perception not only of the mechanisms but of the people they affect. It can also be expected to inform and perhaps also transform behavioral approaches to tobacco cessation therapy.
Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking. A national perspective. JAMA. 1990 Sep 26;264(12):1541-5.
Anderson HR, Cook Dr. Passive smoking and sudden infant death syndrome: Review of the epidemiologic evidence. Thorax 1997;52:1003–1009.
Campaign for Tobacco-Free Kids. Raising cigarette taxes reduces smoking, especially among kids (and the cigarette companies know it). Available from the World Wide Web at www.tobaccofreekids.org.
Carmelli D, Swan GE, Robinette D, Rabsitz R. Genetic influences on smoking—a study of male twins. N Engl J Med 1992;327:829–833.
Centers for Disease Control and Prevention. Achievements in public health, 1900–1999: Tobacco use—United States. MMWR 1999;48:986–993.
Centers for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and economic costs—United States, 1995–1999. MMWR 2002;51:300-303.
Centers for Disease Control and Prevention. Cigarette smoking among adults— United States, 1999. MMWR 2001;50:869–873.
Centers for Disease Control and Prevention. Medical-care expenditures attributable to cigarette smoking during pregnancy—United States, 1995. MMWR 1997;46:1048–1050.
Centers for Disease Control and Prevention. State Highlights 2001: Investment in Tobacco Control. Atlanta: Centers for Disease Control and Prevention, 2001.
Centers for Disease Control and Prevention. Tobacco use among middle and high school students—United States, 1999. MMWR 2000;49:49–53.
Charlton A, Melia P, Moyer C. A manual on tobacco and young people for the industrialized world. International Union Against Cancer (UICC). Geneva, Switzerland, 1990.
Dempsey DA, Benowitz NL. Risks and benefits of nicotine to aid smoking cessation in pregnancy. Drug Safety 2001;24:277–322.
Eysenck HJ. The causes and effects of smoking. Beverly Hills, CA: Sage Publications, 1980.
Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization. Addict Behav 1978;3:235-46.
Fisher RA. Cigarettes, cancer and statistics. Centennial Review 1958;2:151–166.
Floyd Rl, Rimer BK, Giovino Ga, Mullen PD, Sullivan Se. A review of smoking in pregnancy: effects on pregnancy outcomes and cessation efforts. Annu Rev Public Health 1993;14:379–411.
Gilpin E, Choi, WS, Berry C, Pierce JP. How many adolescents start smoking each day in the United States? J Adolesc Health 1999;25:248–255.
Hall W, Madden P, Lynskey M. The genetics of tobacco use: methods, findings and policy implications. Tobacco Control 2002;11:119–124.
Haustein KO. Cigarette smoking, nicotine and pregnancy. Int J Clin Pharmacol Therapeutics 1999;37:417–427.
Henningfield JE, Blayton R, Pollin W. Involvement of tobacco in alcoholism and illicit drug use. Br J Addict 1990;85(2):279–291.
Hughes JR. Genetics of smoking: a brief review. Behav Ther 1986;17:335–345.
Johnston LD, O'Malley PM, Bachman JG. National survey results on drug use from the Monitoring the Future Study, 1975–1998. Vol. 1: Secondary school students. Rockville, MD: National Institutes of Health, National Institute on Drug Abuse, 2000.
Kendler KS, Thornton LM, Pedersen NL. Tobacco consumption in Swedish twins reared aparts and rearer together. Arch Gen Psychiatry 2000;57:886– 892.
King C, Siegel M. The Master Settlement Agreement with the tobacco industry and cigarette advertising in magazines. N Engl J Med 2001;345(7):504– 511.
Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol 1996;20:115–126.
McNeill AD, West RJ, Jarvis M, Jackson P, Bryant A. Cigarette withdrawal symptoms in adolescent smokers. Psychopharmacology 1986;90:533-6.
National Cancer Institute. Health effects of exposure to environmental tobacco smoke: The report of the California Environmental Protection Agency. Bethesda, MD: National Cancer Institutes, 1999.
Niu T, Chen C, Ni J, et al. Nicotine dependence and its familial aggregation in Chinese. Int J Epidemiol 2000;29:248–252.
Poswillo D, Alberman E. Effects of smoking on the fetus, neonate, and child. New York: Oxford University Press, 1992.
Prokhorov AV, Pallonen UE, Fava JL, Ding L, Niaura R. Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav 1996;21:117–27.
Prokhorov AV, Koehly LM, Pallonen UE, Hudmon KS. Adolescent nicotine dependence measured by the modified Fagerström Tolerance Questionnaire at two time points. Journal of Child and Adolescent Substance Abuse 1998:21:117-27.
Prokhorov AV, De Moor C, Pallonen UE, Hudmon KS, Koehly L, Hu S. Adolescent nicotine dependence measured by the modified Fagerström tolerance questionnaire with salivary cotinine among adolescents. Addict Behav 2000; 25:429-432.
Schiff I, Bell WR, Davis V, Kessler CM, Meyer C, Nakajima S, Sexton BJ. Oral contraceptives and smoking, current considerations: recommendations of a consensus panel. Am J Obstet Gynecol 1999;180:S383–4.
Spitz MR, Shi H, Yang F, Hudmon KS, Jiang H, Chamberlain RM, Amos CI, Wan Y, Cinciripini P, Hong WK, Wu X. Case-control study of the D2 dopamine receptor gene and smoking status in lung cancer patients. J Natl Cancer Inst 90:358-63, 1998.
Texas Department of Health. Texas Youth Tobacco Survey, 1998. Available on the World Wide Web at www.tdh.state.tx.us/otpc/stats/statistics.htm#98txyts.
Texas Department of Health. Texas Youth Tobacco Survey, 1999. Available on the World Wide Web at www.tdh.state.tx.us/otpc/stats/statistics.htm#99txyts.
U.S. Department of Health and Human Services. Women and smoking: a report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2001.
U.S. Department of Health and Human Services. Daily reasons to breathe clean. Washington, D.C.: Office on Women's Health, USDHHS. On the World Wide Web at www.4woman.gov/QuitSmoking/reasons_list.cfm.